Abstract
Introduction: Diamond Blackfan anemia (DBA) is an inherited bone marrow failure syndrome (IBMFS) characterized by hypoplastic anemia, congenital malformations and an increased risk to develop malignancies.Until now, treatment of DBA consists of red blood cell (RBC) transfusions, glucocorticoids (GC) and allogeneic hematopoietic stem cell transplantation in a selection of patients. Whereas RBC transfusions are the main cause of IO, elevated iron parameters have also been reported in non-transfusion-dependent DBA patients. Here we investigated the incidence and severity of IO in a well-described cohort of transfusion-dependent and non-transfusion-dependent DBA patients in order to gain more insight in the regulation of iron metabolism in DBA, and to provide clinical guiding to improve the diagnosis and management of IO in DBA.
Methods: In this retrospective, observational study we have included twenty-nine pediatric and adult DBA patients for whom at least one serum ferritin level and/or MRI result was available. Ten patients (34%) were classified as transfusion-dependent (TD) (ten or more transfusions during the twelve months prior to evaluation). Non-transfusion-dependent (NTD) patients (66%) were treated with either GC, incidental transfusions or received no treatment. Transfusion burden (transfusion history) was assessed via medical records. Serum ferritin levels ≥250 ng/mL in males and ≥150 ng/mL in females were considered to be elevated. Results of MRI were expressed as liver iron content (LIC) and as cardiac T2* in milliseconds (ms). LIC ≥3 mg/g indicates significant hepatic IO, and LIC ≥7 mg/g is associated with clinical morbidity. Cardiac T2* ≤20 ms indicates significant cardiac IO.
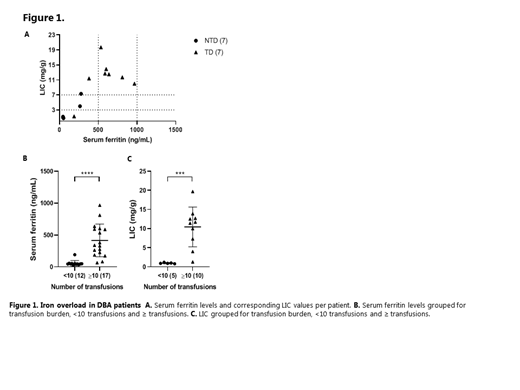
Results: In 15/29 (52%) MRI analysis of IO was performed. Hepatic IO (LIC >3 mg/g) was present in 9/29 (31%) of DBA patients, of which 8/9 (89%) had moderate to severe IO (LIC>7mg/g), despite the fact that all but one were treated with chelation therapy. Overall serum ferritin levels and LIC correlated significantly (r=0.7907, p<0.001), and all TD patients with LIC ≥7 mg/g had serum ferritin levels ³400 ng/mL, however, none of the patients had a serum ferritin >1000 ng/mL (Figure 1A). Interestingly, in the NTD group, hepatic IO was present in 2/7 patients (29%), who both only had mildly elevated serum ferritin levels (263 ng/mL and 277 ng/mL) and were not treated with iron chelation therapy. Based on total transfusion burden since birth, patients were classified in distinct groups: nine patients who received ³10 transfusions during life (9/10) were diagnosed with hepatic IO, whilst none of the patients who received <10 transfusions were diagnosed with hepatic IO. Both mean serum ferritin levels and mean LIC values were significantly higher in patients with ³10 transfusions compared to all with <10 transfusions (Figure 1B-C).
Discussion: We demonstrate that IO is common in DBA yet can be easily overlooked in NTD patients that were treated with transfusions in the past. While serum ferritin levels significantly correlated with LIC values, this parameter cannot be used exclusively to screen for IO or titrate iron chelation therapy. We conclude that in clinical practice, biochemical parameters in combination with transfusion history justify a low-threshold to perform an MRI-based evaluation of IO, and to start adequate chelation therapy.
Van Beers: Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Novartis: Research Funding; RR Mechatronics: Research Funding. Wijk: Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Axcella health: Research Funding; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal